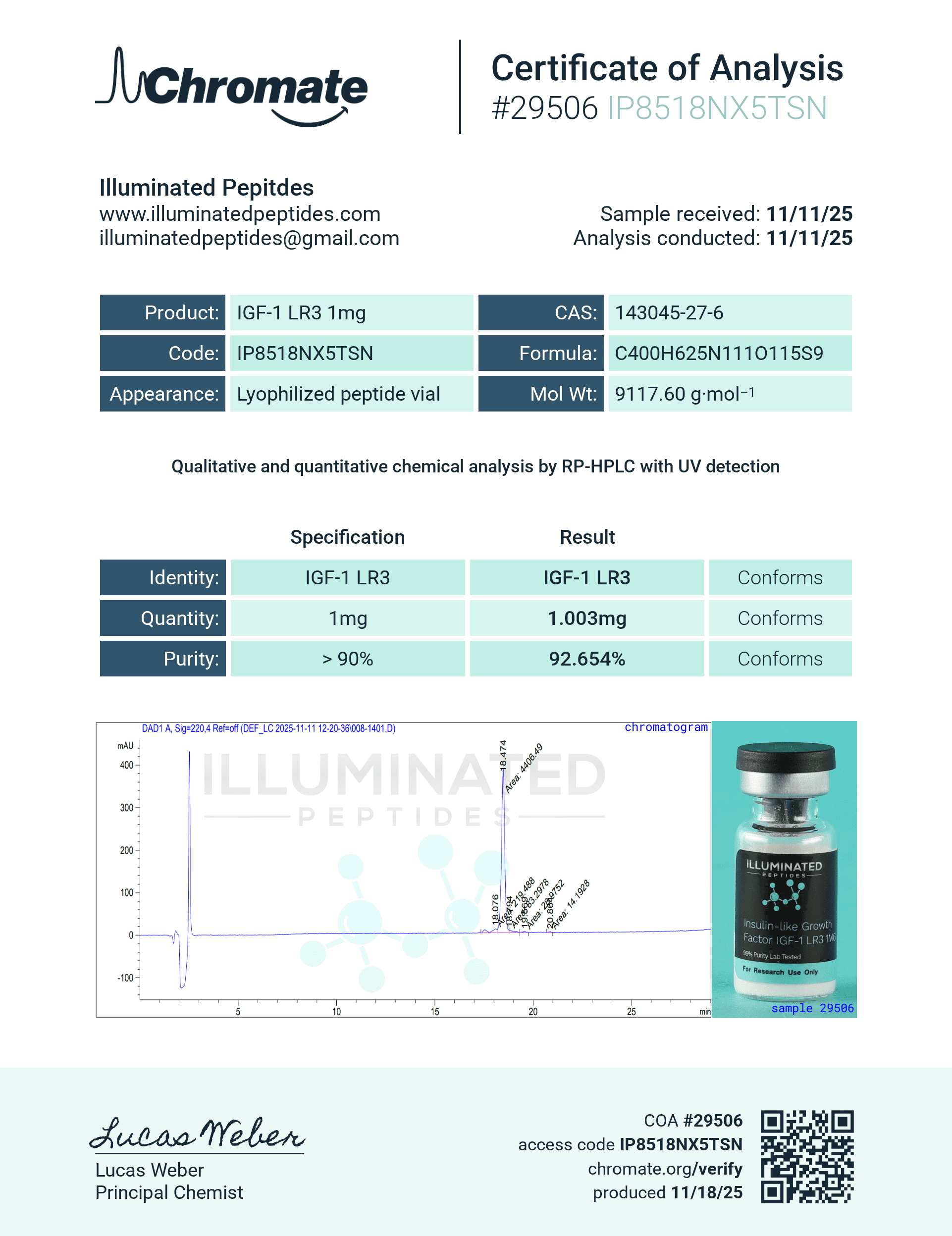
Description
What is IGF-1 LR3 ?
Insulin-like Growth Factor-1 Long R3 (IGF-1 LR3) is a synthetic peptide hormone, modified from natural IGF-1 by substituting arginine at position 3 and extending its amino acid chain. This alteration reduces its binding to IGF-binding proteins, allowing it to remain active in circulation far longer than native IGF-1. By extending its half-life, IGF-1 LR3 enhances cellular growth and division, supports fat metabolism, and promotes muscle repair and hypertrophy by inhibiting myostatin. Research also suggests potential roles for IGF-1 LR3 in supporting tissue regeneration and metabolic balance across different systems.
Oral supplementation of IGF-1 LR3 is considered ineffective, as the peptide is broken down in the gastrointestinal tract before absorption. While experimental sublingual or precursor-based methods have been explored, current evidence shows they do not significantly increase circulating IGF-1 LR3 to functional levels. Additionally, natural declines in growth factor activity with age further limit the effectiveness of oral alternatives. The most reliable results are achieved through direct injection, which ensures stable dosing, predictable bioavailability, and measurable physiological effects.
IGF-1 LR3 Structure
Molecular Formula: C400H625N111O115S9
Molecular Weight: ~9117 g/mol
Sequence Length: 83 amino acids (compared to 70 in native IGF-1)
CAS No: 946870-92-4
Alternative Names: Insulin-like Growth Factor-1 Long R3, IGF-1 LR3, Long Arg3 IGF-1
IGF1-LR3 Research
Cell Division
IGF-1 LR3 is strongly linked to enhanced cell proliferation due to its ability to activate the IGF-1 receptor and downstream signaling pathways, particularly the PI3K-Akt and MAPK cascades. By promoting these pathways, IGF-1 LR3 stimulates DNA synthesis and accelerates the rate of cell division in various tissues. This effect is most notable in muscle, connective tissue, and organ systems that rely on rapid regeneration.
In experimental studies, IGF-1 LR3 has been shown to support tissue repair by encouraging both hyperplasia (increase in cell number) and hypertrophy (increase in cell size). This dual mechanism allows it to play a critical role not only in muscle growth but also in broader regenerative medicine applications, where cell turnover is essential for healing and recovery.
Fat Metabolism and Diabetes
IGF-1 LR3 influences fat metabolism by improving insulin sensitivity and enhancing glucose uptake in muscle cells. Its activation of the insulin receptor and cross-talk with IGF-1 pathways results in more efficient use of carbohydrates and reduced fat storage. This shift in metabolic balance favors lean tissue development over adiposity.
Research has also highlighted the potential of IGF-1 LR3 in supporting individuals with insulin resistance or type 2 diabetes. By improving glucose utilization and reducing circulating blood sugar levels, IGF-1 LR3 mimics some of the beneficial effects of insulin without directly replacing it. These findings point to possible therapeutic roles in metabolic disorders, though more controlled human studies are still required.
Impairs Myostatin
One of the most important actions of IGF-1 LR3 is its ability to counteract myostatin, a natural protein that inhibits muscle growth. By reducing the influence of myostatin, IGF-1 LR3 creates an environment where muscle hypertrophy can proceed more efficiently, allowing for greater gains in strength and size. This effect makes it of high interest in both athletic performance and medical recovery.
Animal models demonstrate that blocking myostatin, either directly or through IGF-1 signaling, results in dramatically increased muscle mass. IGF-1 LR3 enhances protein synthesis and reduces protein degradation, amplifying the muscle-building response while limiting catabolic processes.
In therapeutic contexts, this property may be valuable for patients with muscle-wasting conditions such as sarcopenia, cachexia, or muscular dystrophy. By impairing myostatin activity, IGF-1 LR3 provides a pathway for restoring muscle mass and improving functional strength in clinical populations.
From a mechanistic perspective, the impairment of myostatin by IGF-1 LR3 is achieved through its downstream activation of Akt and mTOR pathways, which are critical for anabolic signaling. This not only increases muscle size but also contributes to enhanced recovery following injury or surgery, highlighting its multifaceted role in muscle health.
IGF-1 LR3 Longevity Research
Emerging evidence suggests that IGF-1 LR3 may influence the biology of aging by supporting tissue maintenance and cellular repair. Higher activity of IGF-1 has been associated with improved bone density, better cardiovascular function, and protection against age-related decline in muscle. These effects suggest IGF-1 LR3 could be useful in mitigating some aspects of frailty.
However, longevity research presents a complex picture. While moderate IGF-1 signaling supports vitality, excessively high levels may promote unwanted cell proliferation, potentially raising risks of cancer or accelerated aging processes. Current studies emphasize that balance is key—controlled IGF-1 LR3 activity could aid healthy aging, but long-term impacts remain under investigation.
Glucocorticoid Signaling
Glucocorticoids, such as cortisol, are stress hormones that promote muscle breakdown and impair recovery when chronically elevated. IGF-1 LR3 helps counteract these catabolic effects by activating anabolic pathways that oppose glucocorticoid signaling. This protective mechanism allows for preservation of muscle tissue under conditions of stress or corticosteroid exposure.
Research indicates that IGF-1 LR3 reduces glucocorticoid-induced apoptosis in muscle and bone cells, preserving both structure and function. By doing so, it enhances resilience against conditions like chronic stress, overtraining, or medical treatments that involve glucocorticoids.
Furthermore, the balance between glucocorticoids and IGF-1 signaling may play an important role in overall metabolic health. By tipping the scale toward anabolism, IGF-1 LR3 supports recovery, repair, and long-term tissue stability despite the presence of catabolic hormonal influences.