Description
What Is BPC-157?
BPC-157, short for Body Protection Compound-157, is a synthetic fragment derived from the naturally occurring body protection compound (BPC), a protein present in the human gastrointestinal system. BPC is known to contribute to maintaining the structural integrity of the digestive lining and has been studied for its influence on vascular development and tissue resilience.
The laboratory-produced peptide BPC-157 is a pentadecapeptide consisting of 15 amino acids that originate from the larger BPC protein sequence. Research indicates that this smaller fragment retains several of the biological activities observed in the parent compound. Experimental studies have examined its effects on:
- Tissue repair and regeneration
- Angiogenesis (formation of new blood vessels)
- Modulation of the coagulation process
- Regulation of nitric oxide pathways
- Immune system responses
- Cellular gene expression
- Neurohormonal activity within the gastrointestinal tract
BPC-157 Structure
Sequence: Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val
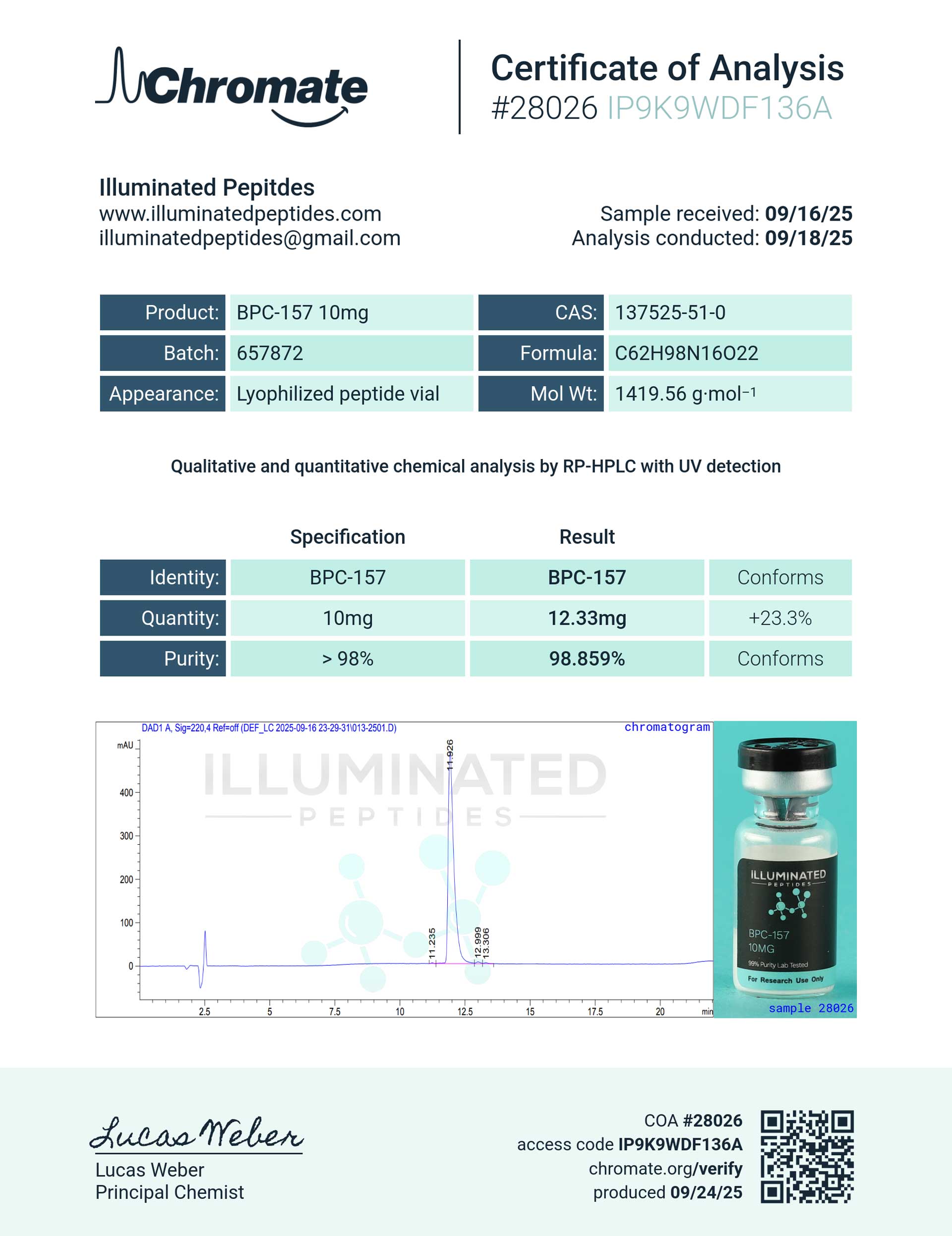
Molecular Formula: C₂H₉N₁₆O₂₂
Molecular Weight: 1419.556 g/mol
PubChem CID: 108101
BPC-157 Research
BPC-157 and Wound Healing
Within the gastrointestinal tract, the naturally occurring body protection compound (BPC) is thought to play a role in preserving mucosal barrier integrity, shielding underlying tissues from exposure to digestive fluids such as gastric acid and bile. Experimental models suggest that BPC-157 may influence fibroblast behavior, with observed dose-dependent effects on their proliferation and migration. Fibroblasts are essential for wound repair as they produce extracellular matrix proteins including collagen, fibrin, and elastin, all of which contribute to tissue remodeling and structural support.
Vascular Growth and Collateralization
Studies indicate that BPC-157 demonstrates pro-angiogenic properties by enhancing the proliferation of endothelial cells, the primary cells lining blood vessels. In animal research, it has been shown to promote collateral vessel formation under ischemic conditions, primarily within the gastrointestinal system, but with indications of similar outcomes in cardiovascular, neurological, and muscular tissues. Such findings suggest its potential as a probe for studying tissue recovery and vascular biology. Further evidence from embryonic models points to stimulation of VEGFR2, a receptor involved in nitric oxide signaling pathways that regulate endothelial growth, proliferation, and survival, as one possible mechanism underlying these angiogenic effects.
Cell culture investigations have shown that BPC-157 can stimulate a process often referred to as vascular “running,” in which new vessels extend toward injured regions or circumvent blockages to help maintain circulation and preserve cellular function. This experimental observation highlights the peptide’s potential role as a probe for studying collateralization and vascular adaptation under conditions of restricted blood flow. Research into these mechanisms may provide valuable insights into how blood vessels respond to occlusion and could inform future strategies in the field of vascular biology.
BPC-157 and Tendon Healing
Due to its observed influence on fibroblast activity and vascular growth, BPC-157 has been widely studied in relation to tendon, ligament, bone, and other connective tissues. These tissues are known to heal slowly, primarily because of their limited blood supply, which delays the arrival of fibroblasts and other repair-associated cells. In both in vitro and in vivo research with rat tendons, BPC-157 has been shown to support collateral vessel development and increase fibroblast density within injured connective tissue. Comparative studies suggest that its activity may surpass that of factors such as bFGF, EGF, and VEGF in experimental models.
Further investigations using FITC-phalloidin staining demonstrate that BPC-157 strongly stimulates F-actin formation in fibroblasts. Since F-actin plays a central role in maintaining cell structure and enabling migration, this finding highlights a potential mechanism for enhanced cellular activity. Western blot analysis has also revealed that BPC-157 increases phosphorylation of paxillin and FAK proteins, both of which are integral to the signaling pathways that regulate cell migration.
Antioxidant Properties
Animal studies suggest that BPC-157 may help regulate oxidative stress by influencing markers such as nitric oxide and malondialdehyde (MDA). These findings point toward potential antioxidant activity, further supported by data showing reduced generation of reactive oxygen species within the gastrointestinal system. Additional experiments using engineered Lactococcus lactis bacteria as a delivery vector demonstrated a significant rise in BPC-157 levels in cell culture, highlighting ongoing interest in alternative methods of administration for studying its biological activity.
BPC-157 and Drug Side Effects
One of the major challenges in pharmaceutical research is balancing therapeutic action with the potential for adverse effects. For example, nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen are limited in long-term use due to risks like gastric irritation and cardiovascular strain. The concept of reducing unwanted reactions while preserving desired outcomes is a central pursuit in experimental medicine. Preclinical findings suggest that BPC-157 may influence pathways associated with drug-related side effects, including those linked to NSAIDs, certain psychiatric agents, and cardiovascular compounds, making it a valuable peptide of interest for ongoing scientific investigation.
It is well established that BPC-157 supports gastrointestinal protection in experimental settings, but studies suggest its influence may extend to other organ systems as well. In rodent models, administration of BPC-157 has been associated with protection against QTc prolongation, a condition that can trigger arrhythmias and is often linked to certain antidiabetic and psychiatric compounds. Beyond cardiovascular effects, research also indicates that BPC-157 may mitigate neurological side effects of psychiatric agents, such as catalepsy and sensory disturbances. These findings highlight the peptide’s broad potential in laboratory investigations of how experimental compounds interact with multiple tissues and signaling pathways.
BPC-157 and Bees
Colony collapse disorder (CCD) describes the sudden decline of entire honey bee populations, a phenomenon that poses major concerns for pollination and food production. While the exact causes remain under investigation, one contributing factor appears to be gastrointestinal infection by the fungus Nosema ceranae. Experimental studies have explored supplementing bee diets with BPC-157 and observed reduced fungal damage in the digestive tract, alongside improved hive survival in field conditions. These findings represent the first evidence that oral delivery of BPC-157 may offer a protective effect against stressors contributing to CCD, providing a valuable model for understanding pollinator health.
Future BPC-157 Research
BPC-157 continues to be widely studied in both cell culture and animal models, where it is being examined not only for its role in supporting tissue repair and vascular regulation but also as a valuable research tool for exploring the underlying biology of these processes. In particular, its impact on angiogenesis—a process central to wound repair, embryonic development, and tumor progression—makes it an important peptide for advancing scientific understanding. Ongoing studies suggest that BPC-157 offers insights that extend well beyond regenerative biology, providing researchers with new avenues to investigate growth and cellular adaptation.
BPC-157 has shown minimal reported side effects in preclinical models, with moderate oral uptake and strong subcutaneous bioavailability in mice. It is important to note that animal dosing cannot be directly applied to humans. BPC-157 offered for purchase is strictly limited to laboratory and educational research purposes only. This compound is not approved for human consumption, and access is restricted to qualified research professionals.